Since 2013, significant scientific research has been conducted to evaluate the efficacy of supplementation with specific micronutrients in managing eye floaters. In five clinical trials European researchers demonstrated notable improvements in patients suffering from vitreous floaters when supplemented with VitroCap®N. This food supplement contains 5 mg of zinc, 40 mg of vitamin C, 26.3 mg Grape seed extract (25 mg of proanthocyanidins), 100 mg of Citrus fruit extract (60 mg of biflavonoids as Hesperidin) and 125 mg of L-lysine.

A simplified schematic representation illustrating normal and clumped collagen fibers in the vitreous body, based on E. Ankamah, J.M. Nolan, and L.C. Huang’s work.
Title of study: FLIES (Floater Intervention Study)
Title of publication: Dietary Intervention With a Targeted Micronutrient Formulation Reduces the Visual Discomfort Associated With Vitreous Degeneration
(Ankamah E et al (2021) Translational Vision Science and Technology, 10(12):19)
Research Team: Emmanuel Ankamah, Marina Green-Gomez, Warren Roche, Eugene Ng, Ulrich Welge-Lüßen, Thomas Kaercher, and John M. Nolan
Trial Overview: The FLIES study is the first to assess the impact of targeted nutritional supplementation on vitreous floaters, in the context of a randomized, double-blind, placebo-controlled clinical trial. The clinical trial included 61 patients, aged 18-79 years, who were supplemented with VitroCap®N. The evaluation was conducted using the following measures:
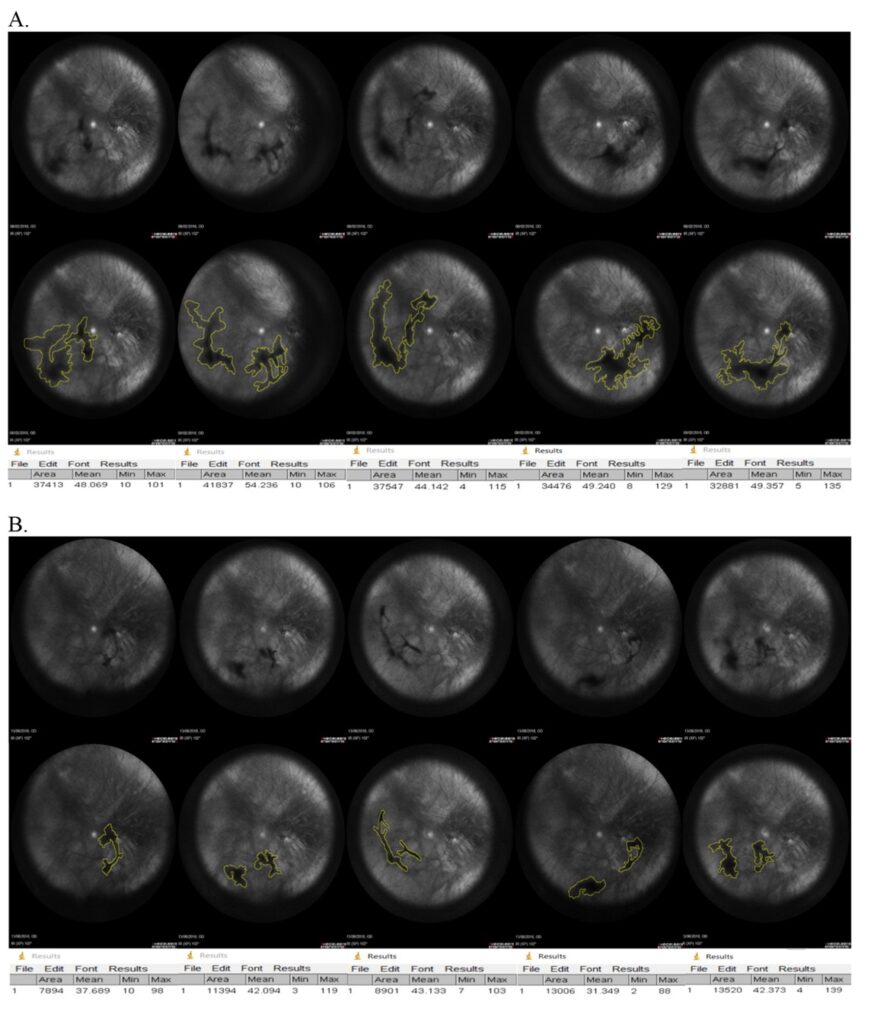
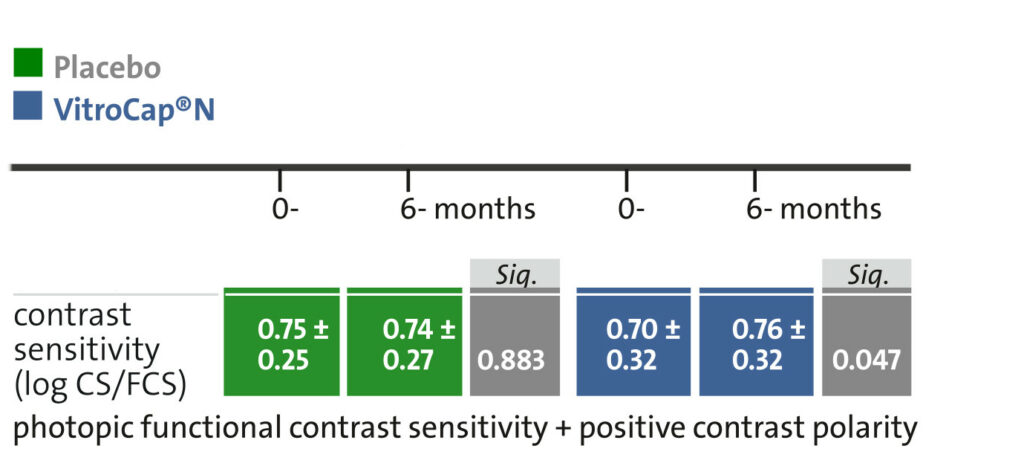
Results: The research was able to demonstrate significant improvements in both subjective visual disturbances and in objective parameters.
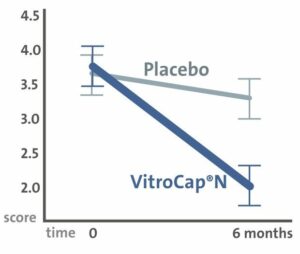
2. The vitreous opacity was reduced in 77% of the patients.
3. A significant improvement in visual function was observed concerning the contrast sensitivity.
The observed benefit in the active group confirms that dietary intake of a formulation of antioxidative and antiglycative micronutrients could mitigate the mechanisms underlying vitreous degeneration, thereby decreasing the visual discomfort associated with vitreous floaters.
2024 update: Warren Roche, PhD, lead statistician of the FLIES study, will leverage his statistical expertise to delve deeper into the original findings and explore their clinical implications within this new study. More information to be found here: https://www.opticianonline.net/content/news/vivaquity-and-ebiga-vision-launch-new-floater-study
Title of publication: Efficacy of “Vitrocap” in patients with vitreous degeneration
(Varganova TS et al (2019) ОФТАЛЬМОФАРМАКОЛОГИЯ [OPHTHALMOPHARMAKOLOGIE], 12, 67-72.)
Research team: Т.S. Varganova, А.G. Veryasova and Е.D. Erysheva
Trial overview: The main aim of the trial was to evaluate the clinical efficacy of nutritional supplementation in patients with vitreous degeneration. Additionally, the psychological characteristics of individuals complaining of ‘floaters’ were analyzed. The clinical trial was conducted with 32 patients aged 37-57 years, all of whom had vitreous floaters confirmed by ocular ultrasonography. Patients were divided into two groups: 16 who were supplemented with VitroCap® for 3 months (main group) and 16 who received a placebo (control group). The evaluation was carried out using the following measures:
Results: The research was able to demonstrate significant improvements in subjective visual disturbances (Questionnaire) and in the objective parameters (standard ocular ultrasonography) after treatment with VitroCap®.
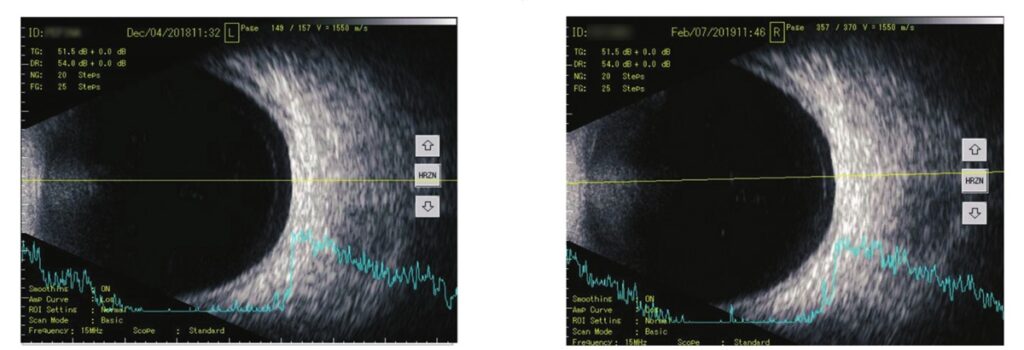
The observations confirmed that VitroCap® supplement improves visual comfort and, in most cases, reduces patients’ subjective complaints of floaters. Additionally, it was demonstrated that after treatment with VitroCap®, there was a tendency for a reduction in vitreous opacities and changes in their qualitative characteristics.
Title of publication: The Effect of oral Supplementation with L-lysine, Hesperidin, Proanthocyanidins, Vitamin C and Zinc on Subjects’ Assessment of the Quality of Vision in Patients with Vitreous Floaters
(Sobol M et al (2018) Journal of Alzheimer’s Disease, 64 (3),1034.)
Research team: Maria Sobol, Maciej Osęka, Thomas Kaercher and Bettina Schmiedchen
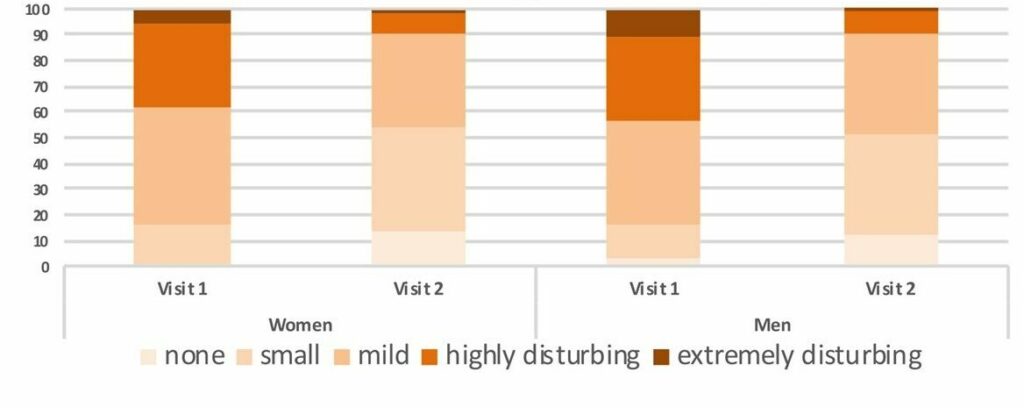
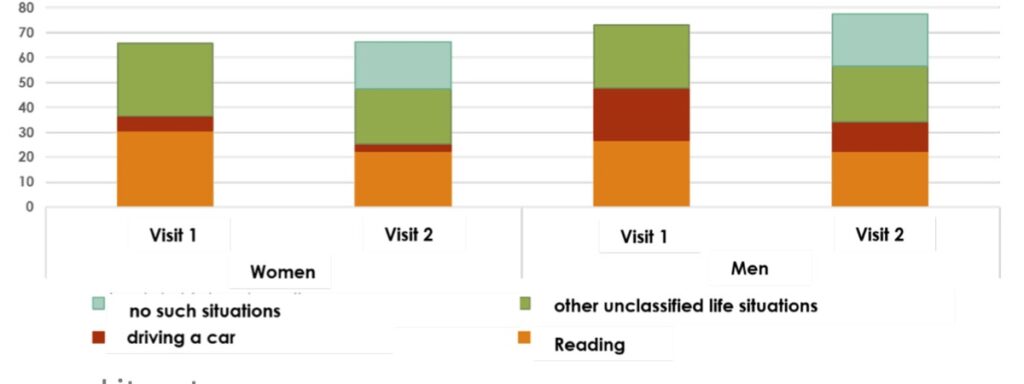
Trial overview: The observational trial was performed with 463 patients, aged 15-61 years. Their visual floaters symptoms were verified before treatment: 90% of the patients reported visual disturbances. They were mild in 27%, moderate in 28%, highly disturbing in 28% and extremely disturbing in 7% at baseline. For the analysis, the patients were divided according to their gender, and within each sex in addition to the age ranges of 20-30, 30-40, 40-50, 50-60, 60-70, 70-80 and 80 respectively. Evaluation was conducted before and after 3 months of treatment with the VitroCap®N-formulation using the following measures:
Results: The research was able to demonstrate significant improvements in subjective visual disturbances and disturbing situations.
Title of publication: Experience of VitroCap® use in vitreous degeneration
(Marchenko L et al (2015) ОФТАЛЬМОЛОГИЯ Восточная Европа [OPHTHALMOLOGY Eastern Europe], 2 (25),123-128.)
Research Team: L.N. Marchenko, A.A. Dalidovich, T.V. Kachan, I.G. Gudievskaya, N.V. Nikitina and M.G. Lonskaya
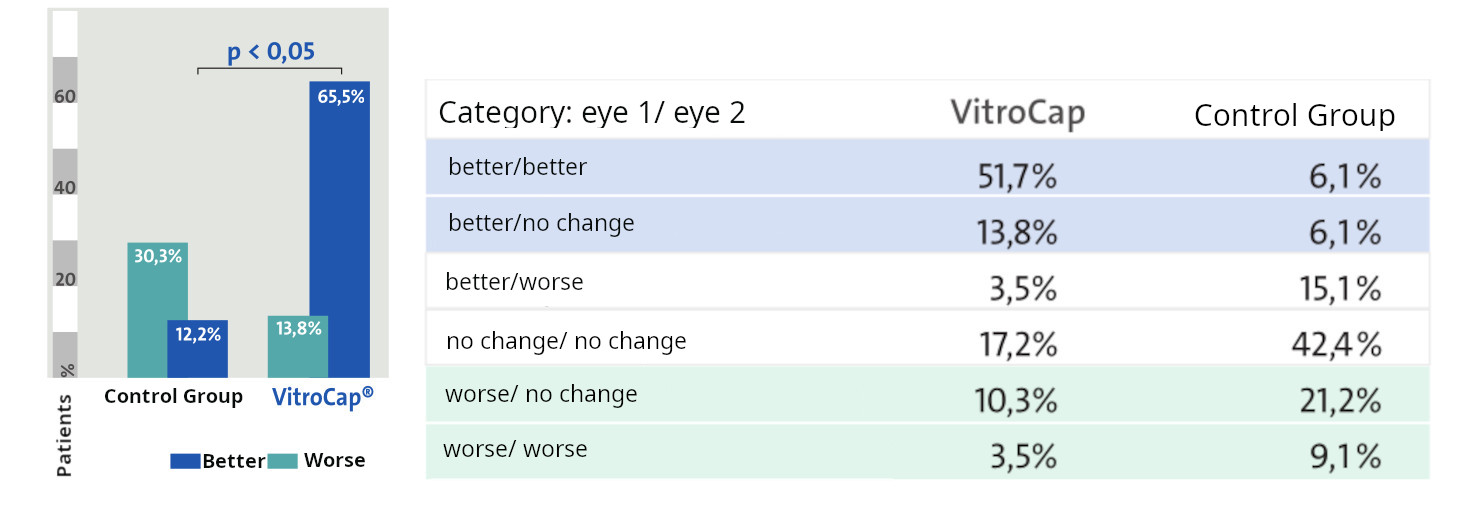
Trial Overview: The clinical trial involved 62 patients aged 40-64 years to assess the efficacy of VitroCap® in treating vitreous degeneration. Confirmation of vitreous floaters was achieved via ocular ultrasonography. Patients were divided into two groups: the treatment group (Group I) comprised 29 patients (58 eyes) who received one capsule of VitroCap® daily with a meal, while the control group (Group II) comprised 33 patients (66 eyes) who did not receive any treatment for vitreous degeneration. Both groups were similar in terms of age and medical parameters. Evaluation was conducted three months post-treatment.
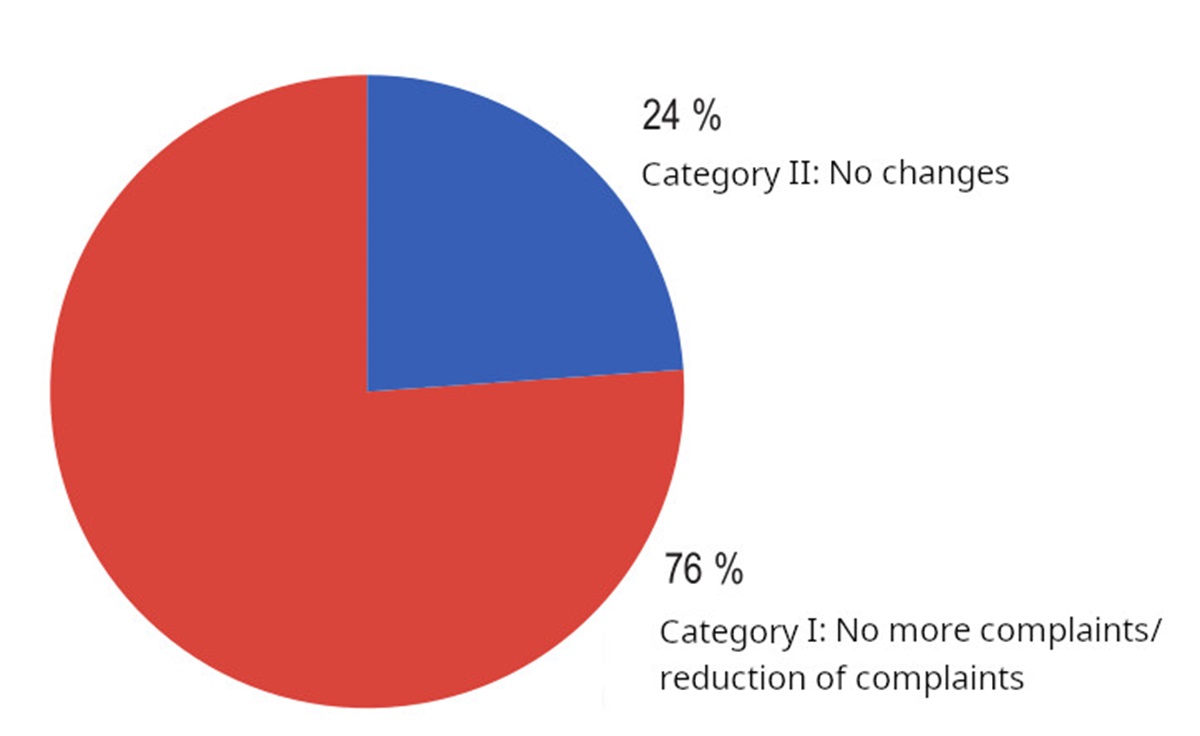
Results: After the administration of VitroCap®, 65.5% of patients reported an improvement in vision quality. In contrast, the control group exhibited a 12.4% improvement over the three-month period. Changes observed in slit-lamp examination and B-mode vitreous ultrasonography results were comparable between both groups. Additionally, no significant changes were detected in the function of the visual system.
Title of publication: With dietary means against an annoying visual disturbance -Observation of application results in symptom improvement Mouches volantes after nutritional influence on the vitreous metabolism
⇓ DOWNLOAD ARTICLE IN JOURNAL OF PRACTICAL OPHTALMOLOGY & OPHTAMOLOGICAL EDUCATION
(Gerste RD and Kaercher T (2013) Zeitschrift für praktische Augenheilkunde & Augenärztliche Fortbildung, 344, Pharma Report April 2013)
⇓ DOWNLOAD POSTER TRIALS 2013 & 14
Research Team: Dr. Th. Kaercher
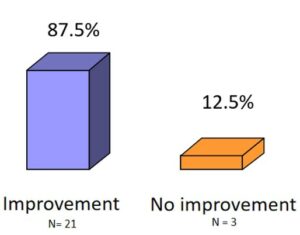
Trial Overview: 24 patients participated in the observation, ranging in age from 21 to 81 years. Vitreous body floaters were confirmed using slit lamps. More than 80% of the participants reported noticing disturbances in their field of vision. All patients were treated with VitroCap® for 3 months. Subjective floater disturbance before and after Treatment was assessed using:
Results: The number of disturbing floater effects after intake was reduced in the morning, in the evening and while reading. Improved after the supplementation with VitroCap® was affirmed by 87,5% of patients. Overall visual disturbance scar decreased from 3,63 to 1,33.